Publications
Teratology Primer, 3rd Edition
What Is the Timeline of Important Events During Pregnancy that May Be Disrupted by a Teratogenic Exposure?
Steven B. Bleyl 1 and Gary C. Schoenwolf 2
Departments of Pediatrics 1 and Neurobiology and Anatomy 2,
University of Utah School of Medicine
Salt Lake City, Utah, USA
Clinicians, and “expecting” parents, typically envision pregnancy as consisting of three sequential trimesters, each lasting three months. Many events occur during prenatal development that are critical for the success of the pregnancy and, ultimately, the birth of a healthy child. Thus, embryologists and teratologists identify milestones of pregnancy (periods and phases) during which essential events occur. Normal development of the conceptus—the zygote, blastocyst, embryo, and fetus, depending on its stage of development, plus its supporting membranes and placenta—can be adversely affected by poor maternal health and nutrition, genetic mutation, exposures to exogenous agents or a combination of these factors. The focus of this chapter is on the timing of important events during pregnancy that may be disrupted by a teratogenic exposure (Fig. 1). The conceptus is susceptible to such exposures throughout in utero development and even postnatally, although there are critical periods in which the conceptus is highly susceptible that are dependent on the endpoint that is affected by the exposure. Moreover, the conceptus is conceived during fertilization as the male and female gametes—the sperm and egg—join together in the upper end of the oviduct (Fallopian tube) to form a single-cell zygote. Consequently, environmental exposures over the life span of the prospective mother or father (both prenatally and postnatally; see discussion of gametogenesis below) can impact the ability of their gametes to produce a successful pregnancy and the birth of a healthy child. Thus, susceptibility to teratogen exposure for a given conceptus actually begins even prior to when it is conceived.
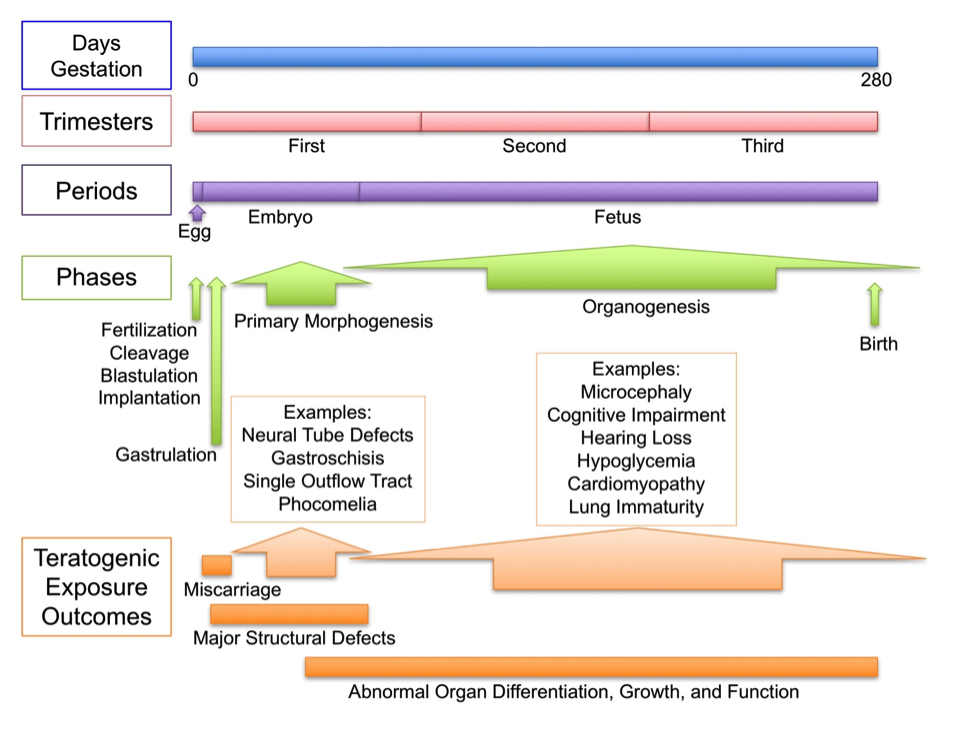
Figure 1. Timeline of important events during pregnancy that may be disrupted by a teratogenic exposure. Shown are the lengths of pregnancy in days (0-280, from conception or fertilization to birth), the span of the three trimesters (3 months each), the three periods of prenatal development (egg, embryo, and fetus), key developmental events (fertilization, cleavage, implantation, gastrulation, primary morphogenesis, organogenesis, and birth), and some outcomes of teratogenic exposure.
In addition to using trimesters to measure the progress of pregnancy, three periods of development are used as milestones: the period of the “egg,” the period of the embryo, and the period of the fetus. The period of the egg is generally defined as the time during pregnancy that precedes implantation—that is, the time from formation of the zygote until the blastocyst burrows into the wall of the receptive, that is, hormonally primed, uterus (initiated by the end of the first week postfertilization). Although the conceptus at this stage is not truly an egg or oocyte, as traditionally defined, for all intents and purposes it looks like an egg, being grossly spherical, throughout this first period. The second period, the period of the embryo, is roughly defined as the time from implantation through the 8th week of development. During the period of the embryo, part of the conceptus takes on the shape of what can be readily recognized as an embryo (Fig. 2), a simple organism containing a rudimentary head, trunk, and tail, projecting limb buds, a beating heart, obvious eyes, and a primitive segmentation. The remainder of the conceptus contributes to the so-called extraembryonic membranes, including the amnion, which enclose and protect the embryo during its development, and the fetal component of the placenta. The final period, the period of the fetus, extends from the beginning of the 9th week of gestation until birth. This period is characterized by rapid growth of the fetus and the differentiation of cells, resulting in the formation of distinct tissue types that become assembled into functional organ systems.
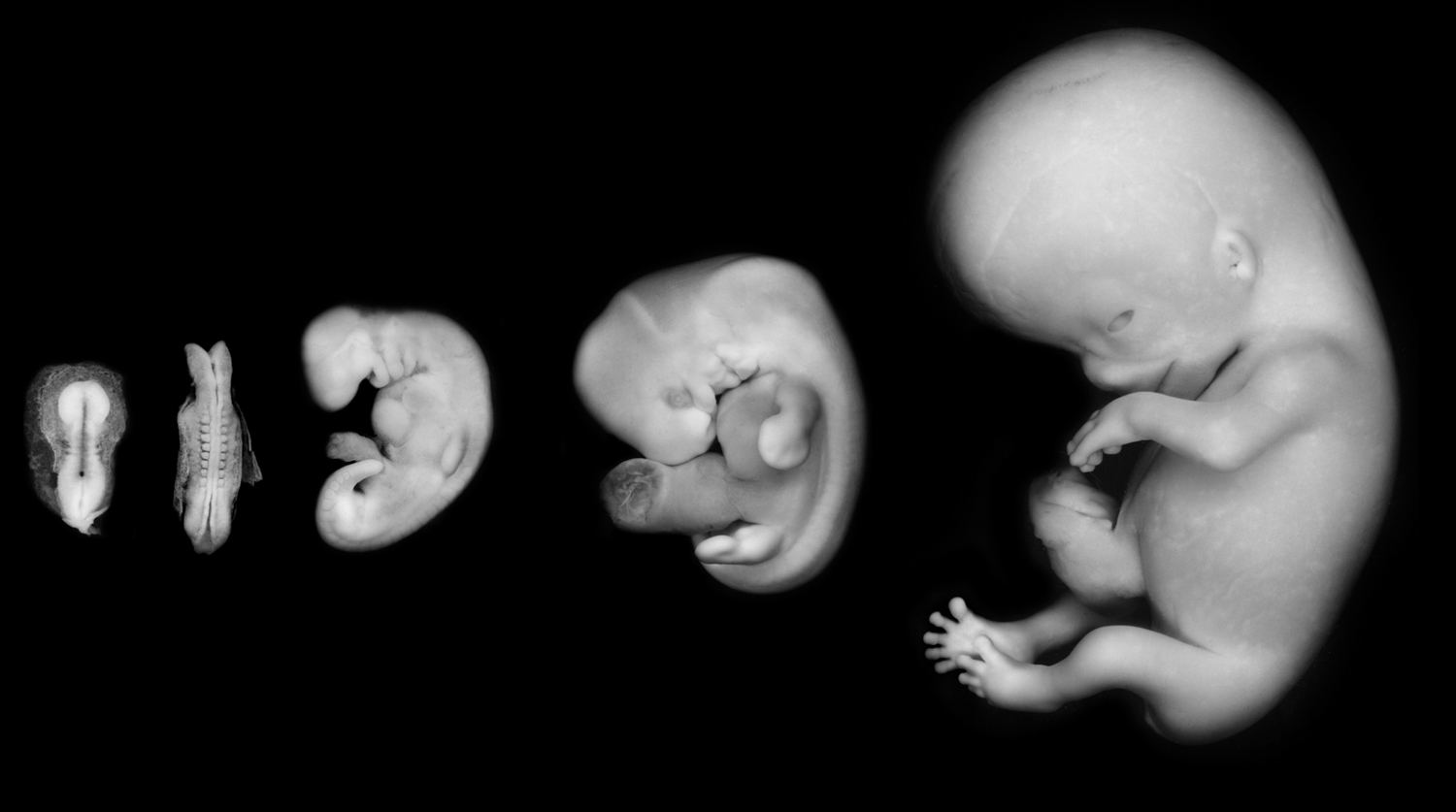
Figure 2. Photographs of human embryos at five stages of gestation reproduced from the collection of the Congenital Anomaly Research Center, Kyoto University Graduate School of Medicine, courtesy of Dr. Kohei Shiota, Ms. Chigako Uwabe, and Dr. Shigehito Yamada. Shown (from left to right) are Carnegie Stages 9, 10, 13, 17, and 23 during the period of the embryo. The Stage 9 embryo has initiated neurulation, which is largely completed by Stage 10 with the exception that the cranial and caudal ends of the neural tube remain open as the neuropores. By Stage 13, body folding has established the tube-within-a-tube body plan of the early embryo, with a distinct head, trunk, and tail, and paddle-like limb buds. By Stage 17, the developing eyes are readily identifiable, and the limb buds now have bulbous distal plate-like structures that will form the hands and the feet. By Stage 23—the last stage in the period of the embryo—all external structures have taken on morphologies similar to those of the adult.
Periods of development broadly define the structure of the developing organism at three different times during pregnancy. For example, the “egg” (used in the sense of the developing organism present during the period of the egg) contains the precursor cells that will form the embryo itself and will contribute to the supporting tissues necessary for the embryo’s development. However, the structure of the “egg” is rather different, and not intuitively linked, to the definitive structure of the embryo proper and its supporting tissues, which are elaborated from the “egg” during the period of the embryo. The embryo contains the rudiments of the organs of the fetus; the link between the structure of the embryo and fetus is more intuitive, but, for example, the paddle-like limb buds present in the early embryo are non-functional and have a very different structure than that of the upper and lower limbs of the fetus, which at birth are fully functional. Just as the egg contains the precursor cells for the rudiments of the embryo, the embryo contains the precursor cells for all of the tissues and organs of the fetus. Precursor cells—regardless of their period in development—are highly susceptible to disruption by teratogenic exposures, which are capable of adversely altering their survival, rate of proliferation, migratory activity, ability to differentiate, or to function properly.
In contrast to periods of development, phases of development define not the structure of the developing organism, but the unique developmental events that are occurring at that time. Four major phases are recognized in the prenatal development of humans: gametogenesis; fertilization, cleavage, and blastulation; gastrulation and formation of the tube-within-a-tube body plan; and organogenesis, with cellular and tissue differentiation and rapid growth. The gametes are generated during gametogenesis in the ovaries of the female and the testes of the male. During gametogenesis, germ cells (from each of the prospective parents as they were developing in utero—first identifiable in the yolk sac, an extraembryonic membrane broadly attached to the ventral side of the early embryo) migrate to the developing gonads, divide mitotically, and then initiate meiosis, which is completed postnatally after the onset of puberty, resulting in the generation of haploid eggs and sperm.
During the second phase, fertilization, cleavage, and blastulation, the egg and sperm produced during gametogenesis fuse soon after coitus (or shortly after in vitro fertilization is initiated) to produce a diploid zygote, which rapidly begins a series of mitotic divisions (that is, undergoes cleavage) to produce a solid ball of cells called a morula. As the morula passes down the oviduct toward the uterus, it forms an internal cavity, transforming into a hollow cyst-like structure called the blastocyst. The latter is capable of implanting into the wall of the uterus, initiating in utero development of the embryo. During implantation, the blastocyst differentiates two cell regions: the outer trophoblast, consisting of the cells that invade the uterine wall and contribute to the formation of the placenta, and the inner cell mass, the source of the embryo and its extraembryonic membranes.
As implantation is occurring, the embryo initiates the third phase, gastrulation, a process in which three distinct cell layers (the germ layers) form, each of which gives rise to specific derivatives. For example, the ectoderm forms the nervous system, the mesoderm forms most of the muscle and bone of the embryo, and the endoderm forms the lining of the gut (gastrointestinal tract). The three germ layers established during gastrulation consist of three uniform layers stacked upon each other like pancakes. This essentially two-dimensional stack becomes sculpted into a three-dimensional embryo having a tube-within-a-tube body plan and containing rudiments of all of the major organ systems. How this body plan is achieved is referred to as primary morphogenesis and it involves localized changes in the shape, size, position, and numbers of cells in the three germ layers, generating tissue movements such as thickening, folding, delamination, and fusion.
As a result of primary morphogenesis, specific organ rudiments are generated as well as an embryonic body that is now largely separated from its surrounding extraembryonic membranes. For example, the outer tube of the tube-within-a-tube body plan is the future body wall; it is generated by the expansion, folding, and fusion of the edges of the two-dimensional embryo. Its outer wall is in direct contact with amniotic fluid after formation of the amniotic cavity. Simultaneously, the inner tube of the tube-within-a-tube body plan is formed: the future gut tube, lined with endoderm. As these processes are occurring, other primary morphogenetic events generate the rudiments of the major organ systems. For example, during a process called neurulation, a portion of the ectoderm thickens, folds inward, and fuses at its edges to form a third tube, the neural tube, the rudiment of the entire adult central nervous system; some cells left over from the process of neurulation delaminate from the ectoderm to form neural crest cells, an important population of migratory cells that contribute to a number of organ systems including the mesenchyme of the developing face, the cranial and spinal ganglion of the peripheral nervous system, and the septum that partitions the outflow tract of the heart into two major vessels that separate the systemic and pulmonary circulation: the aortic and pulmonary vessels. Mesodermal cells in the developing trunk of the embryo undergo segmentation to form transient structures called somites, which give rise to the muscles and bones of the trunk and the adjacent dermis of the skin. Some cranial mesodermal cells condense into paired rudiments that rearrange to form tubes that fuse beneath the developing cranial gut and form the heart during the process of cardiogenesis. After formation a single heart tube, this rudiment rapidly loops upon itself, begins to beat and pump blood, and then partitions to form four rudimentary chambers: the right and left atria, and right and left ventricles. Still other mesodermal cells arrange into tubules, contributing to the developing urogenital system.
The final phase, organogenesis, involves the growth and differentiation of precursor cells and tissues contained within each of the organ rudiments formed during primary morphogenesis. Of the four phases, organogenesis occurs over the longest period of time, extending from about four weeks of development (during the period of the embryo), throughout the fetal period, and for some organ systems even continuing postnatally.
Teratogenic exposure during any period or phase of development can have dire consequences (Fig. 1). In general, disruption of the earliest developmental stages (gametogenesis; fertilization, cleavage, and blastulation) results in the loss of the conceptus (that is, a miscarriage, often before the woman realizes she is pregnant). Disruption somewhat later during primary morphogenesis and organogenesis often results in major structural anomalies (a “birth defect” for example, a neural tube defect, such as spina bifida; a ventral body wall defect, such as gastroschisis; a heart defect, such as the formation of a single outflow tract; a limb anomaly, such as phocomelia; or a facial cleft, such as cleft lip or palate). Disruption during the late embryonic and fetal period generally results in abnormal organ differentiation, growth, and function (for example, cognitive impairment, hearing loss, neonatal hypoglycemia, lung immaturity). Thus, the timing of a particular teratogenic exposure can result in drastically different outcomes.
Three examples that illustrate this concept, discussed in more detail in other chapters, are maternal diabetes, maternal alcohol consumption during pregnancy, and maternal infection (and subsequently fetal infection) with Zika virus. Early gestational diabetes can result in a wide range of structural birth defects, including neural tube defects, cardiac septation defects, renal anomalies, and growth restriction, whereas late-onset gestational diabetes can result in increased growth of the fetus (large for gestational age), neonatal hypoglycemia, and abnormal differentiation of certain organs (for example, outflow tract obstruction of the heart due to septal hypertrophy). Maternal consumption of alcohol during the early embryonic period can cause a recognizable pattern of structural birth defects affecting the brain, facial appearance, and growth. Consumption of alcohol later in pregnancy is thought to cause impaired cognition and behavioral disorders, without obvious structural anomalies. Finally, maternal infection with Zika virus, either transmitted to her through an infectious mosquito bite or through her partner’s semen during intercourse up to several months after he received an infectious mosquito bite, can affect fetal development throughout the three trimesters of pregnancy. Infection during the first trimester may lead to miscarriage, whereas during the second and third trimesters, the most likely teratogenic outcomes are microcephaly and mental retardation.
References
Carlson BM. 2014. Human Embryology and Developmental Biology. 5th ed. Philadelphia: Elsevier Mosby. 520 p.
Moore KL, Persaud T.V.N., Torchia, M.G. 2016. The Developing Human. Clinically Oriented Embryology. 10th ed. Philadelphia: Elsevier Saunders. 560 p.
O'Rahilly R, Müller F. 1987. Developmental Stages in Human Embryos. Washington, D.C.: Carnegie Institution of Washington. 306 p.
Sadler TW. 2014. Langman's Medical Embryology. 13th ed. Philadelphia: Lippincott Williams & Wilkins. 424 p.
Schoenwolf GC, Bleyl SB, Brauer PR, Francis-West PH. 2014. Larsen's Human Embryology. 5th ed. Philadelphia: Elsevier Churchill Livingstone. 576 p.
